Alaabtayadu waxay galeen liiska UK ee aaladaha ogaanshaha invitro ee coronavirus-ka laga dhaafay!
Waxaad ka hubin kartaa liiska bogga internetka ee Waaxda Caafimaadka UK: https://www.gov.uk/.../medical-devices-regulations-2002... Haddii aad u baahan tahay inaad iibsato alaabtayada, waxaad nagala soo xidhiidhi kartaa at wakhti kasta!
Warbixinta Tijaabada iyo Falanqaynta Silico ee StrongStep® SARS-CoV-2 Tijaabada Degdegga ah ee Antigen ee Kala duwanaanta SARS-CoV-2
SARS-CoV-2 waxay hadda horumarisay dhowr isbedel oo leh cawaaqib xun, qaar sida B.1.1.7, B.1.351, B.1.2, B.1.1.28, B.1.617, oo ay ku jiraan omicron mutant strain (B1.1.529) ayaa la sheegay maalmihii la soo dhaafay.Sida soo saaraha reagent IVD, waxaan had iyo jeer fiiro gaar ah u leh horumarinta dhacdooyinka khuseeya, hubi isbeddelada asiidhyada amino ee khuseeya iyo qiimaynta saamaynta suurtagalka ah ee isbeddellada on reagents.
StrongStep® SARS-CoV-2 Tijaabada Degdegga ah ee Antigen-ka Geli liiska guud ee EU ee nadaafadda iyo badbaadada cuntada
StrongStep® SARS-CoV-2 Tijaabada Degdegga ah ee Antigen Geli liiska guud ee EU-da ee nadaafadda iyo badbaadada cuntada, taas oo ah mid ka mid ah dhowr warshadood oo leh 100% dareenka marka qiimaha CT uu ka yar yahay 25%.
StrongStep® SARS-CoV-2 Tijaabada Degdegga ah ee Antigen-ka ee lagu daray liiska qiimeynta ee HEL
StrongStep® SARS-CoV-2 Tijaabada Degdegga ah ee Antigen-ka ayaa lagu daray liiska qiimeynta FIND.Aasaaska cusub ee ogaanshaha cusub (FIND), waa urur ku takhasusay qiimeynta waxqabadka xirmooyinka ee iskaashiga istiraatiijiyadeed ee WHO.
Warbixin ku saabsan fayraska kala duwan
Falanqaynta isku xigxiga ee falanqaynta ayaa muujisay in goobta isbeddelka ee kala duwanaanshaha SARS-CoV-2 ee lagu arkay Boqortooyada Ingiriiska, Koonfur Afrika iyo Hindiya aysan dhammaantood ku jirin gobolka naqshadeynta ee asaasiga ah iyo baaritaanka hadda.StrongStep® Novel Coronavirus (SARS-CoV-2) Qalabka PCR ee-waqtiga-dhabta-dhabta ah (ogaanshaha saddexda hidde-side) ayaa dabooli kara oo ogaan kara noocyada mutant (oo lagu muujiyey shaxda soo socota) iyada oo aan wax saameyn ah ku yeelan waxqabadka hadda.Sababtoo ah ma jiro wax isbeddel ah oo ku yimid gobolka ee isku xigxiga ogaanshaha.
Soo koobida Warbixinta Qiimaynta ee Machadka Kala duwan ee StrongStep® SARS-CoV-2 Antigen Rapid Test
We have received many certificate or EUA from different countries, such as United Kingdom, Singapore, Brazil, South Africa, Malaysia,Indonesia,Philippine, Argentine, Guatemala and so on. Also, we have send our products to many institue for evaluation, below are the summary of some data. Please contact us via guangming@limingbio.com if you need full artical of following report.
Thailand FDA COVID 19 ATK 2021 T6400429
Dhawaan, StrongStep® SARS-CoV-2 Antigen Rapid Test oo ay soo saartay Nanjing Liming Bio-products Co. Ltd ayaa si guul leh u heshay shahaadada FDA ee Thailand (lambarka diiwaangelinta T 6400429,T 6400430,T 6400431,T 6400432) loo ogolaaday inuu galo suuqa Thailand.
StrongStep® SARS-CoV-2 Antigen Rapid Test ayaa helay xaqiijinta waxqabadka Paul-Ehrlich-Institut (PEI) ee Jarmalka!
Dhawaan, Nanjing LimingBio's Novel Coronavirus (SARS-CoV-2) reagent ogaanshaha antigen "StrongStep® SARS-CoV-2 Antigen Rapid Test" wuxuu helay xaqiijinta waxqabadka Paul-Ehrlich-Institut (PEI *) gudaha Jarmalka, badeecadan ayaa ahayd shahaado ay siisay Wakaaladda Federaalka Jarmalka ee Dawooyinka iyo Maamulka Qalabka Caafimaadka (BfArM).LimingBio waxay noqotay mid ka mid ah soosaarayaasha yar ee Shiinaha ee helay shahaadada labadaba ee BfArM+PEI ee Jarmalka.Tijaabada degdega ah ee antigen-ka ee Liming Bio ayaa ka gudubtay shahaadada awooda leh ee Wasaaradda Caafimaadka ee wadamo badan, taas oo si buuxda u caddaynaysa waxqabadka wanaagsan ee xirmada.
Baaritaanka degdega ah ee antigen-ka SARS-CoV-2
StrongStep® SARS-CoV-2 Antigen Rapid Test waa baaritaan degdeg ah oo immunochromatographic ah oo lagu ogaanayo COVID-19 antigen ilaa fayraska SARS-CoV-2 ee ku jira dhuunta bini'aadamka / naasopharyngeal.
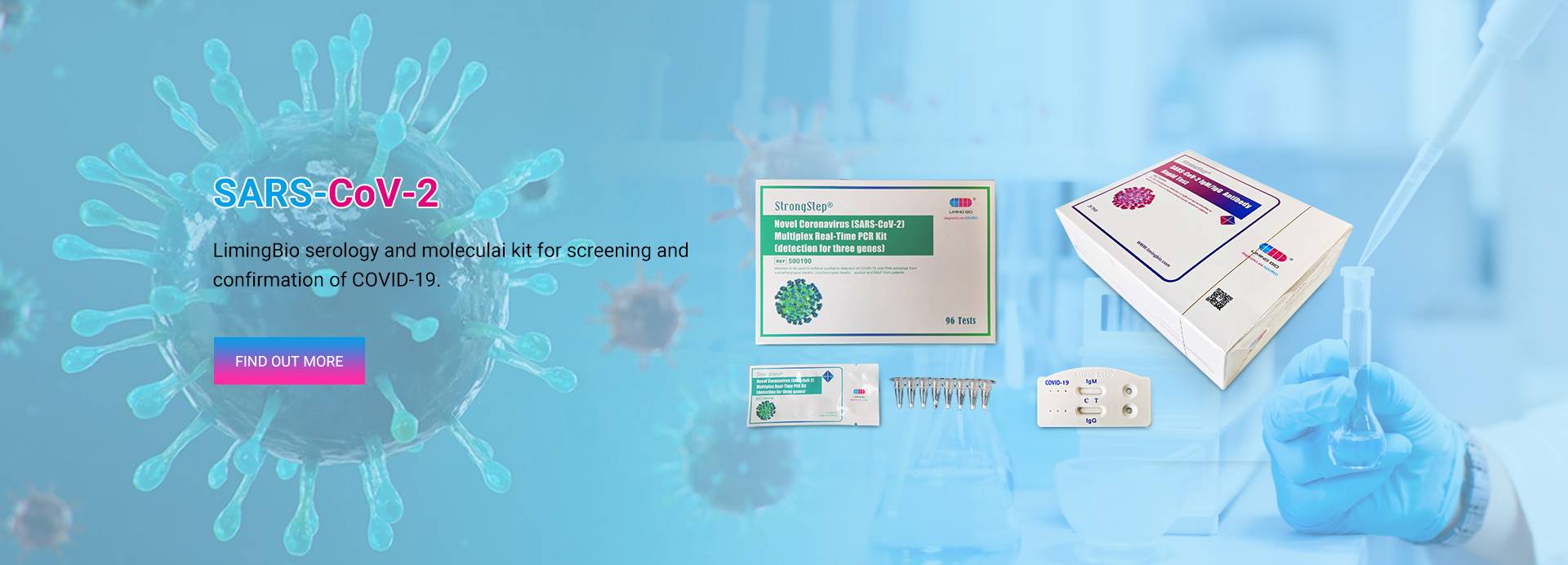
Novel Coronavirus (SARS-CoV-2) Qalabka PCR-waqtiga-dhabta ah ee Multiplex
Xirmadan aadka u xasaasiga ah, ee diyaar u ah in la isticmaalo PCR waxa lagu heli karaa qaab lyophilized ah (habka qalajinta qaboojinta) kaydinta muddada dheer.Qalabka waxa lagu rari karaa oo lagu kaydin karaa heerkulka qolka waana mid deggan muddo hal sano ah.
Agabkeenii ugu dambeeyay
NAGU SAABSAN
Nanjing Liming Bio-products Co., Ltd. oo la aasaasay 2001, shirkadeena waxay ku takhasustay horumarinta, soo saarista iyo suuqgeynta tijaabooyinka degdega ah ee cudurada faafa gaar ahaan STDs.Marka laga reebo ISO13485, ku dhawaad dhammaan badeecadaheena waa calaamad CE iyo CFDA waa la ansixiyay.Badeecadahayadu waxay muujiyeen waxqabad la mid ah marka loo eego hababka kale (oo ay ku jiraan PCR ama dhaqanka) kuwaas oo waqti badan qaata oo qaali ah.Isticmaalka imtixaanadayada degdega ah, ama bukaan-socodka ama xirfadlayaasha daryeelka caafimaadku waxay badbaadin karaan waqti badan sugitaan sababtoo ah waxay u baahan tahay 10 daqiiqo oo keliya.



















1人份抗原卡实物图唾液版1_00_副本-300x216.png)


















