SARS-CoV-2
-
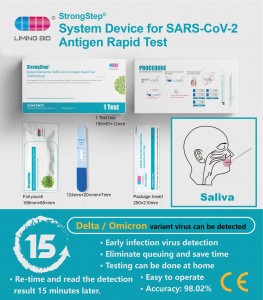
Qalabka Nidaamka Xoogga Howlsstop ee SARS-Cov-2 Tijaabada Degdegga ee Antigen
Nadiifin 500210 Cayimid 1 Imtixaan / Sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin CandhuufAdeegsiga loogu talagalay La jeedashoQalabka Nidaamka ee SARS-Cov-Cov-Tov-Covid Imtixaanka Degdegga ah ayaa shaqaaleysiisa teknolojiyada ImmESOCHRorography si loo ogaado SARS-Cov-2 Nucleocdiid Antige salka cirifka aadanaha. Tijaabadani waa adeegsi keliya oo keliya oo loogu talagalay is-baarista. Waxaa lagu talinayaa in la isticmaalo baaritaankan 7 maalmood gudahood laga bilaabo calaamadaha cudurka. Lt waxaa taageera qiimeynta waxqabadka qaaska. -

SARS-Cov-2 Imtixaan deg deg ah oo Antigen ah (nasal)
Nadiifin 500200 Cayimid 1 Imtixaanno / Sanduuq; 5 Imtixaanno / Sanduuq; 20 Imtixaanno / Sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin Sawxanka saqafka ah ee nasal Adeegsiga loogu talagalay StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. CARRUURTA KELIYA ISTICMAALKA KELIYA oo loogu talagalay is-baarista. Waxaa lagu talinayaa in la isticmaalo baaritaankan 5 maalmood gudahood laga bilaabo calaamadaha cudurka. Waxaa taageera qiimeynta waxqabadka rugta caafimaad. -

SARS-Cov-2 Tijaabada Degdegga ah ee Antigen (Adeegsiga Xirfadeed)
Nadiifin 500200 Cayimid 25 baaritaan / sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin Sawxanka saqafka ah ee nasal Adeegsiga loogu talagalay StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. CARRUURTA KELIYA ISTICMAALKA KELIYA oo loogu talagalay is-baarista. Waxaa lagu talinayaa in la isticmaalo baaritaankan 5 maalmood gudahood laga bilaabo calaamadaha cudurka. Waxaa taageera qiimeynta waxqabadka rugta caafimaad. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
SARS-Cov-2 Imtixaanka Degdega ee Antigen ee candhuufta
Nadiifin 500230 Cayimid 20 baaritaan / sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin CandhuufAdeegsiga loogu talagalay Tani waa deg deg ah oo ka soo baxay fayraska SARS-2 ee Nucleecocdid Nucleocapid Antiven ee Salten-19 ay ku tuhmaan bixiyeha daryeelka caafimaadka shanta maalmood ee bilowga ah ee astaamaha. Asxaabtii waxaa loo isticmaalaa sidii gargaar xagga ogaanshaha cudurka 'Covid-19'. -

Qalabka Nidaamka ee SARS-Cov-2 & Hargabka A / B Combo Antigen Imtixaanka
Nadiifin 500220 Cayimid 20 baaritaan / sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin Sanka / oropharnngnngnngengeal Adeegsiga loogu talagalay Tani waa deg deg ah oo ka soo baxda fayraska SARS-2 ee Nucleecocdi-ka NASAL-ka NASAL / OrmophicnGarngeal-ka bini-aadamka ee laga soo ururiyey shakhsiyaadka daryeelka caafimaadkooda shanta maalmood ee bilowga ah ee astaamaha. Asxaabtii waxaa loo isticmaalaa sidii gargaar xagga ogaanshaha cudurka 'Covid-19'. -

Qalabka Nidaamka Dal SARS ee SARS-Cov-2 Tijaabada Degdega ee Antigen
Nadiifin 500210 Cayimid 20 baaritaan / sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin Sanka / oropharnngnngnngengeal Adeegsiga loogu talagalay Tani waa deg deg ah oo ka soo baxda fayraska SARS-2 ee Nucleecocdi-ka NASAL-ka NASAL / OrmophicnGarngeal-ka bini-aadamka ee laga soo ururiyey shakhsiyaadka daryeelka caafimaadkooda shanta maalmood ee bilowga ah ee astaamaha. Asxaabtii waxaa loo isticmaalaa sidii gargaar xagga ogaanshaha cudurka 'Covid-19'. -

Sheeko-sheeko (SARS-Cov-2) BILAASH MADAXWEYNAHA MADAXWEYNAHA PCR
Nadiifin 500190 Cayimid 96 Imtixaanno / Sanduuq Mabda'a ogaanshaha Tuubbo Tijaabin Nasal / nasopharnngngngeal Swab Adeegsiga loogu talagalay Tan waxaa loogu talagalay in loo isticmaalo ogaanshaha aqoonta ee SARS-Covel rna viral-ka laga soo saaray suuxshiyaha nasopharngeal, xaako iyo balaastigga soo saarista soo saarista ee loo yaqaan 'FDA / CEF's' iyo aaladaha loo yaqaan 'PCR' ee kor lagu soo sheegay. Qalabka waxaa loogu talagalay in lagu isticmaalo shaqaale tababbaran oo sheybaarka ah
-

SARS-Cov-2 & Hargabka A / B Blolemx-ka-waqtiga dhabta ah ee PCR
Nadiifin 510010 Cayimid 96 Imtixaanno / Sanduuq Mabda'a ogaanshaha Tuubbo Tijaabin Nasal / nasopharngngeal Swab / ortophyngngal Adeegsiga loogu talagalay Hardstep® SARS-Cov-2 & Martzaled A / B Bloles-yada Waqtiga-dhabta ah waxaa loogu talagalay ogaanshaha tayada ee PCR-2, infalawansada fayraska ah ee ku jira sanka ee dawladaha daryeelka caafimaadka iyo nanazarygeal ama tufaaxyada suufka ah ee ku dhaca suufka iyo sanka ee is-ururinta ama tufaaxyada isku soo ururinta (lagu soo aruuriyay goob daryeel caafimaad oo ay ku shaqeeyaan bixiye caafimaad oo lagu tuhunsan yahay infekshanka fayraska ee la raacayo
Qalabka waxaa loogu talagalay in lagu isticmaalo shaqaale tababbaran oo sheybaarka ah
-

SARS-Cov-2 Igm / Igm / Igg oo ka hortag ah
Nadiifin 502090 Cayimid 20 baaritaan / sanduuq Mabda'a ogaanshaha ImmESOCHOMOGOGOGOGOGOGOGOGOGOGOGOGOGOGOQA Tijaabin Dhiiga oo dhan / Plasma Adeegsiga loogu talagalay Kani waa fal deg-deg ah oo ka-hortagga ah ee ku saabsan ogaanshaha isku-darka ah ee ku saabsan ogaanshaha isku-darka ee IGM iyo IGG Subagga difaaca jirka ee SARS-2 ee SARS-2 ee dadka bini-aadamka, Serum ama Plasma. Tijaabadu waxay ku kooban tahay Mareykanka si loogu qaybiyo sheybaarada ay caddeeyeen CLIA si ay u fuliyaan imtixaanka kakanaanta sare.
Tijaabadani ma aysan eegin FDA.
Natiijooyinka taban kama horjoogsadaan infakshanka sareeya-2-aad.
Natiijooyinka ka soo baxa baaritaanka ka hortagga ah looma isticmaali karo in lagu garto ama laga saaro cudurka SARS-2 ee Sanad-2.
Natiijooyinka togan ayaa laga yaabaa inay sabab u tahay infekshanka aan la soo koobi karin ama soo bandhigaya kuwa aan SARS-SONONAvirus-ka, sida Coronavirurus Hku1, nl63, OC43, ama 229E.







